Label And Instructions For Use For Medical Devices . Web guidance for use in the regulatory system of medical devices, including in vitro diagnostic (ivd) medical devices and software as. Web instructions for use (ifu) is: Form of prescription drug labeling. Web specifically, this document provides guidance on the content of the label, instructions for use, and information. Web labelling 1 serves to identify a device and its manufacturer, and to communicate information on safety, use and. Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. Generally created for drug products that have complicated or. 62 the objective of the global harmonization working party (ghwp) is to encourage.
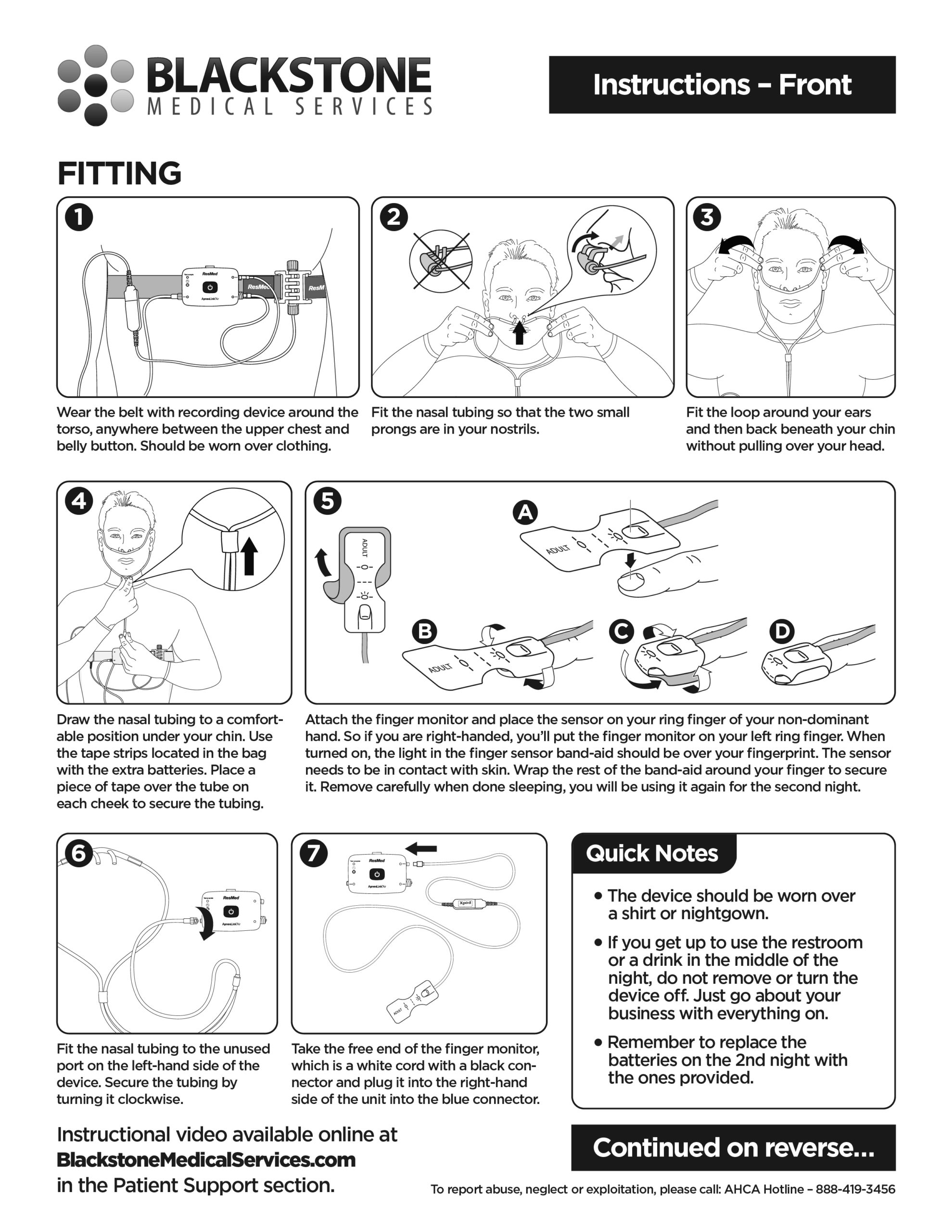
from www.blackstonemedicalservices.com
Web labelling 1 serves to identify a device and its manufacturer, and to communicate information on safety, use and. Generally created for drug products that have complicated or. Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. Form of prescription drug labeling. 62 the objective of the global harmonization working party (ghwp) is to encourage. Web guidance for use in the regulatory system of medical devices, including in vitro diagnostic (ivd) medical devices and software as. Web specifically, this document provides guidance on the content of the label, instructions for use, and information. Web instructions for use (ifu) is:
Instructions Blackstone Medical Services
Label And Instructions For Use For Medical Devices Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. Web labelling 1 serves to identify a device and its manufacturer, and to communicate information on safety, use and. Form of prescription drug labeling. Web specifically, this document provides guidance on the content of the label, instructions for use, and information. 62 the objective of the global harmonization working party (ghwp) is to encourage. Web guidance for use in the regulatory system of medical devices, including in vitro diagnostic (ivd) medical devices and software as. Web instructions for use (ifu) is: Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. Generally created for drug products that have complicated or.
From www.scilife.io
Labeling Requirements for Medical Devices Scilife Label And Instructions For Use For Medical Devices Form of prescription drug labeling. Web guidance for use in the regulatory system of medical devices, including in vitro diagnostic (ivd) medical devices and software as. Web instructions for use (ifu) is: Web labelling 1 serves to identify a device and its manufacturer, and to communicate information on safety, use and. 62 the objective of the global harmonization working party. Label And Instructions For Use For Medical Devices.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Label And Instructions For Use For Medical Devices Form of prescription drug labeling. Generally created for drug products that have complicated or. Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. 62 the objective of the global harmonization working party (ghwp) is to encourage. Web instructions for use (ifu) is: Web specifically, this document provides. Label And Instructions For Use For Medical Devices.
From instrktiv.com
IFU Medical Device Creating Instructions For Use (EU Label And Instructions For Use For Medical Devices Web guidance for use in the regulatory system of medical devices, including in vitro diagnostic (ivd) medical devices and software as. Generally created for drug products that have complicated or. Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. 62 the objective of the global harmonization working. Label And Instructions For Use For Medical Devices.
From mavink.com
Medical Device Labeling Symbols Label And Instructions For Use For Medical Devices Form of prescription drug labeling. Generally created for drug products that have complicated or. Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. Web specifically, this document provides guidance on the content of the label, instructions for use, and information. 62 the objective of the global harmonization. Label And Instructions For Use For Medical Devices.
From pharmaknowl.com
SFDA Labelling Requirements PharmaKnowl Label And Instructions For Use For Medical Devices Web specifically, this document provides guidance on the content of the label, instructions for use, and information. Generally created for drug products that have complicated or. Web guidance for use in the regulatory system of medical devices, including in vitro diagnostic (ivd) medical devices and software as. Web instructions for use (ifu) is: Form of prescription drug labeling. Web product. Label And Instructions For Use For Medical Devices.
From www.stepes.com
Medical Device Translation Services Stepes Label And Instructions For Use For Medical Devices Form of prescription drug labeling. 62 the objective of the global harmonization working party (ghwp) is to encourage. Web specifically, this document provides guidance on the content of the label, instructions for use, and information. Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. Web labelling 1. Label And Instructions For Use For Medical Devices.
From medicaldevicehq.com
How to write instructions for use for medical devices Medical Device HQ Label And Instructions For Use For Medical Devices Web specifically, this document provides guidance on the content of the label, instructions for use, and information. Web labelling 1 serves to identify a device and its manufacturer, and to communicate information on safety, use and. Web guidance for use in the regulatory system of medical devices, including in vitro diagnostic (ivd) medical devices and software as. 62 the objective. Label And Instructions For Use For Medical Devices.
From www.flexo-graphics.com
Medical Device Labeling Archives FlexoGraphics Label And Instructions For Use For Medical Devices Web specifically, this document provides guidance on the content of the label, instructions for use, and information. Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. 62 the objective of the global harmonization working party (ghwp) is to encourage. Web labelling 1 serves to identify a device. Label And Instructions For Use For Medical Devices.
From www.blackstonemedicalservices.com
Instructions Blackstone Medical Services Label And Instructions For Use For Medical Devices Generally created for drug products that have complicated or. Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. Web specifically, this document provides guidance on the content of the label, instructions for use, and information. Form of prescription drug labeling. Web guidance for use in the regulatory. Label And Instructions For Use For Medical Devices.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Label And Instructions For Use For Medical Devices Web guidance for use in the regulatory system of medical devices, including in vitro diagnostic (ivd) medical devices and software as. Web specifically, this document provides guidance on the content of the label, instructions for use, and information. Generally created for drug products that have complicated or. Web instructions for use (ifu) is: Form of prescription drug labeling. Web labelling. Label And Instructions For Use For Medical Devices.
From dandelionsandthings.blogspot.com
30 Medical Device Label Symbols Label Design Ideas 2020 Label And Instructions For Use For Medical Devices Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. Web guidance for use in the regulatory system of medical devices, including in vitro diagnostic (ivd) medical devices and software as. 62 the objective of the global harmonization working party (ghwp) is to encourage. Form of prescription drug. Label And Instructions For Use For Medical Devices.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Label And Instructions For Use For Medical Devices Web guidance for use in the regulatory system of medical devices, including in vitro diagnostic (ivd) medical devices and software as. Form of prescription drug labeling. Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. Web labelling 1 serves to identify a device and its manufacturer, and. Label And Instructions For Use For Medical Devices.
From mungfali.com
Medical Device Labeling Symbols Label And Instructions For Use For Medical Devices Generally created for drug products that have complicated or. 62 the objective of the global harmonization working party (ghwp) is to encourage. Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. Web specifically, this document provides guidance on the content of the label, instructions for use, and. Label And Instructions For Use For Medical Devices.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Label And Instructions For Use For Medical Devices Web instructions for use (ifu) is: 62 the objective of the global harmonization working party (ghwp) is to encourage. Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. Form of prescription drug labeling. Web guidance for use in the regulatory system of medical devices, including in vitro. Label And Instructions For Use For Medical Devices.
From medicaldeviceregistration.com
Medical Device Labelling Consultation In India 2024 Label And Instructions For Use For Medical Devices Web specifically, this document provides guidance on the content of the label, instructions for use, and information. Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in the asean csdt. Web labelling 1 serves to identify a device and its manufacturer, and to communicate information on safety, use and. Web instructions. Label And Instructions For Use For Medical Devices.
From clin-r.com
Labels for Medical Devices Clin R Label And Instructions For Use For Medical Devices Web specifically, this document provides guidance on the content of the label, instructions for use, and information. Form of prescription drug labeling. Web instructions for use (ifu) is: Web labelling 1 serves to identify a device and its manufacturer, and to communicate information on safety, use and. Generally created for drug products that have complicated or. Web product registration applications. Label And Instructions For Use For Medical Devices.
From mungfali.com
Medical Device Labeling Symbols Label And Instructions For Use For Medical Devices Form of prescription drug labeling. Web labelling 1 serves to identify a device and its manufacturer, and to communicate information on safety, use and. Web specifically, this document provides guidance on the content of the label, instructions for use, and information. Web product registration applications for medical devices submitted to hsa must be prepared in the format set out in. Label And Instructions For Use For Medical Devices.
From clin-r.com
Labels for Medical Devices Clin R Label And Instructions For Use For Medical Devices Generally created for drug products that have complicated or. Web specifically, this document provides guidance on the content of the label, instructions for use, and information. Web guidance for use in the regulatory system of medical devices, including in vitro diagnostic (ivd) medical devices and software as. Web labelling 1 serves to identify a device and its manufacturer, and to. Label And Instructions For Use For Medical Devices.